A solvent-free solution: vacuum-deposited organic monolayers modify work functions of noble metal electrodes
F. Widdascheck, A.A. Hauke, G. Witte
Advanced Functional Materials 29 (2019) 180385
Work function tailoring by organic monolayers is one of several promising approaches to reducing contact resistance between metal electrodes and organic semiconductors in organic electronics devices. In this study, several polar and non-polar phthalocyanines were used to modify the work functions of noble metal electrodes, for both single-crystalline model surfaces and actual polycrystalline electrodes.
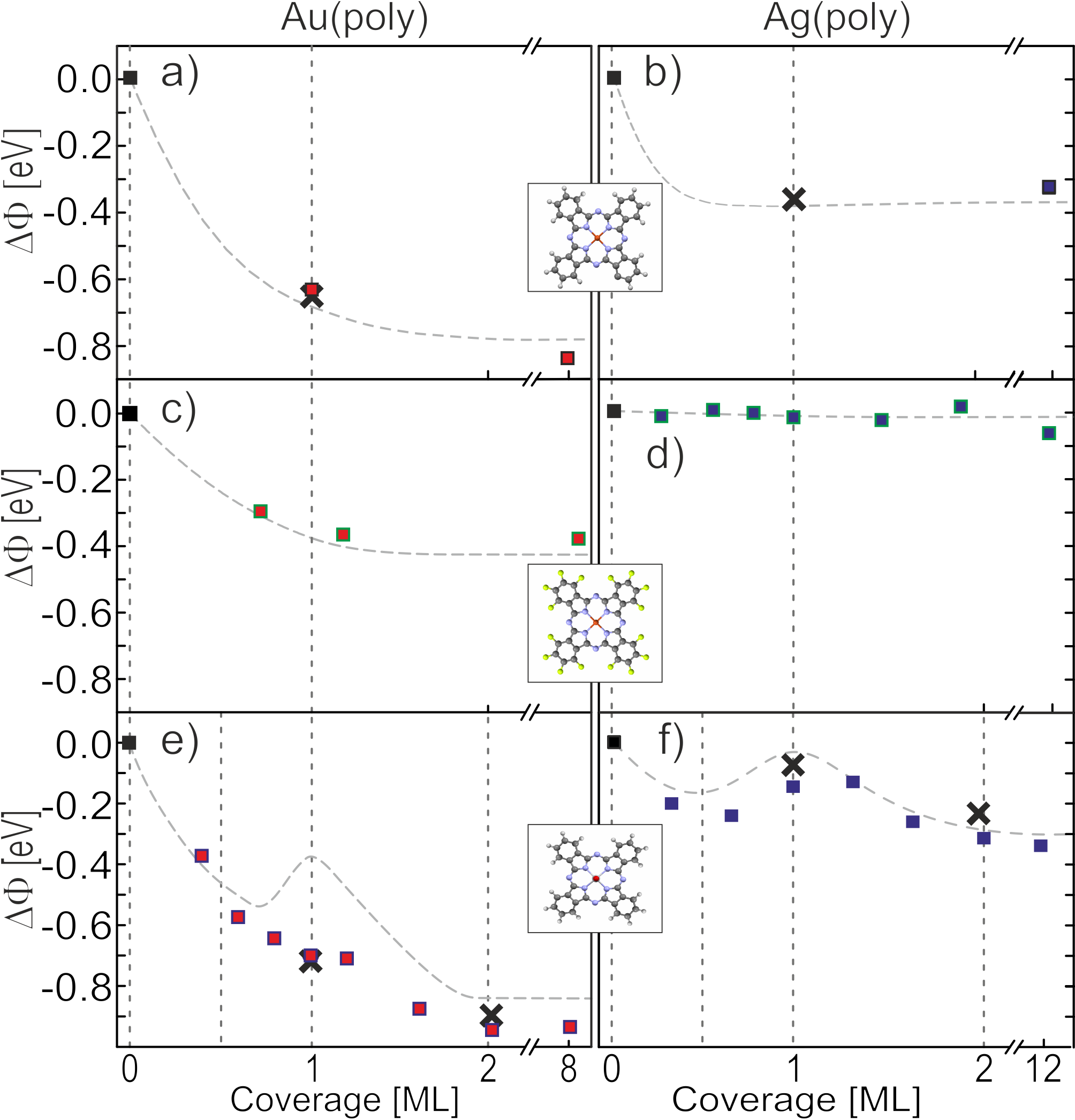
Metal work function changes upon adsorption of a, b) CuPc , c, d) F16CuPc and e, f) TiOPc. Square data points show incremental deposition, crosses refer to multilayer desorption. Dashed grey lines indicate the results for the (111) surfaces.
Atomically flat, ultra-pure single-crystal surfaces allow detailed scanning tunneling microscopy (STM) studies of the adsorption geometry of organic molecules on noble metals. Combining this with an in-situ Kelvin probe setup allows investigation of the change in work function of noble metals as a function of coverage of molecular adsorbates. For the case of phthalocyanines on Au(111) surfaces, a general reduction in work function with increasing molecular coverage can be observed within the first two monolayers due to the formation of interface dipoles between the metal and the phthalocyanine’s carbon backbone. Beyond this coverage, the effect saturates.
The exact extent of this reduction depends on the properties of the chosen molecule: Fluorination for example weakens the effect (observed here for F16CuPc), while the presence of an inherent molecular dipole (e.g. TiOPc) strengthens it. In addition, presence of TiOPc also leads to the formation of a local maximum around the completion of the first monolayer, which is due to the interplay between interface dipoles and molecular dipoles. Similar observations can be made for Ag(111), where the overall reduction is, however, far less pronounced and in the case of F16CuPc not evident at all.

140×140 nm2 STM micrographs of TiOPc bi-(a-c) and monolayer (d) films on Au(111), prepared by multilayer desorption.
Kelvin probe analysis on the type of polycrystalline Au and Ag surfaces relevant for device applications reveals an analogous behavior. Here, higher surface roughness and energetic disorder leads to an altogether reduced effect. For both poly- and single-crystalline metal surfaces, it further appears to be possible to form well-defined monolayers of non-fluorinated phthalocyanines simply by thermal desorption of previously deposited multilayers, such that only the more strongly bound monolayer remains on the substrate. The work functions of thus-produced contact primers match those of the as-deposited monolayers in all cases. For polar phthalocyanines, it is even possible to produce a well-defined bilayer due to interaction of the permanent molecular dipoles. These dipoles further allow differentiation between monolayer- and bi-/multilayer coverage by simple water contact angle measurements. Lastly, this method allows exposure of the phthalocyanine multilayers to the air, then form well-ordered monolayers in vacuum by thermal desorption which exhibit the same properties as contact primers not exposed to air.

Water contact angle in ambient conditions: a) and c) TiOPc bilayer prepared by multilayer desorption, b) and d) TiOPc monolayer prepared by multilayer desorption.






